NEW
SSG-542B-E1CR36H

- メーカー
- Supermicro
- CPU
- Xeon6 6700PXeon6 6700E
- 筐体サイズ
- 4U
4U UP フロント/リアローディングストレージサーバー、36基のホットスワップ対応3.5インチSAS/SATAベイ(SASハードウェアRAID)およびPCIe 5.0搭載
NEW

4U UP フロント/リアローディングストレージサーバー、36基のホットスワップ対応3.5インチSAS/SATAベイ(SASハードウェアRAID)およびPCIe 5.0搭載
NEW

コンパクトなファンレスエッジAIプラットフォーム。6コアのJetson Orin™ NXモジュール搭載。最大117 TOPS。
NEW
M1U-2800-2AF0G2.png)
NEW

NEW

HPC/AIサーバー - AMD EPYC™ 9005/9004 - 8Uデュアルプロセッサー AMD Instinct™ MI355X
NEW

WinFast WS855は、ワークステーション内で最大2基のトリプル幅または2基のダブル幅GPUをサポートする強力なGPUアーキテクチャを搭載。これにはNVIDIA® RTX PRO™ 6000 Blackwell Max-Q Workstation Edition 2基/ RTX 6000/5000/4500 Ada Generation、またはRTX PRO™ 6000 Blackwell Workstation Edition 1基を含む構成が可能です。これにより、AI、高性能コンピューティング、3Dレンダリングアプリケーションの要求に応えるためのパフォーマンス拡張を実現します。
NEW

AI、デザイン、シミュレーション、メディア向け UP ワークステーション
NEW

NEW
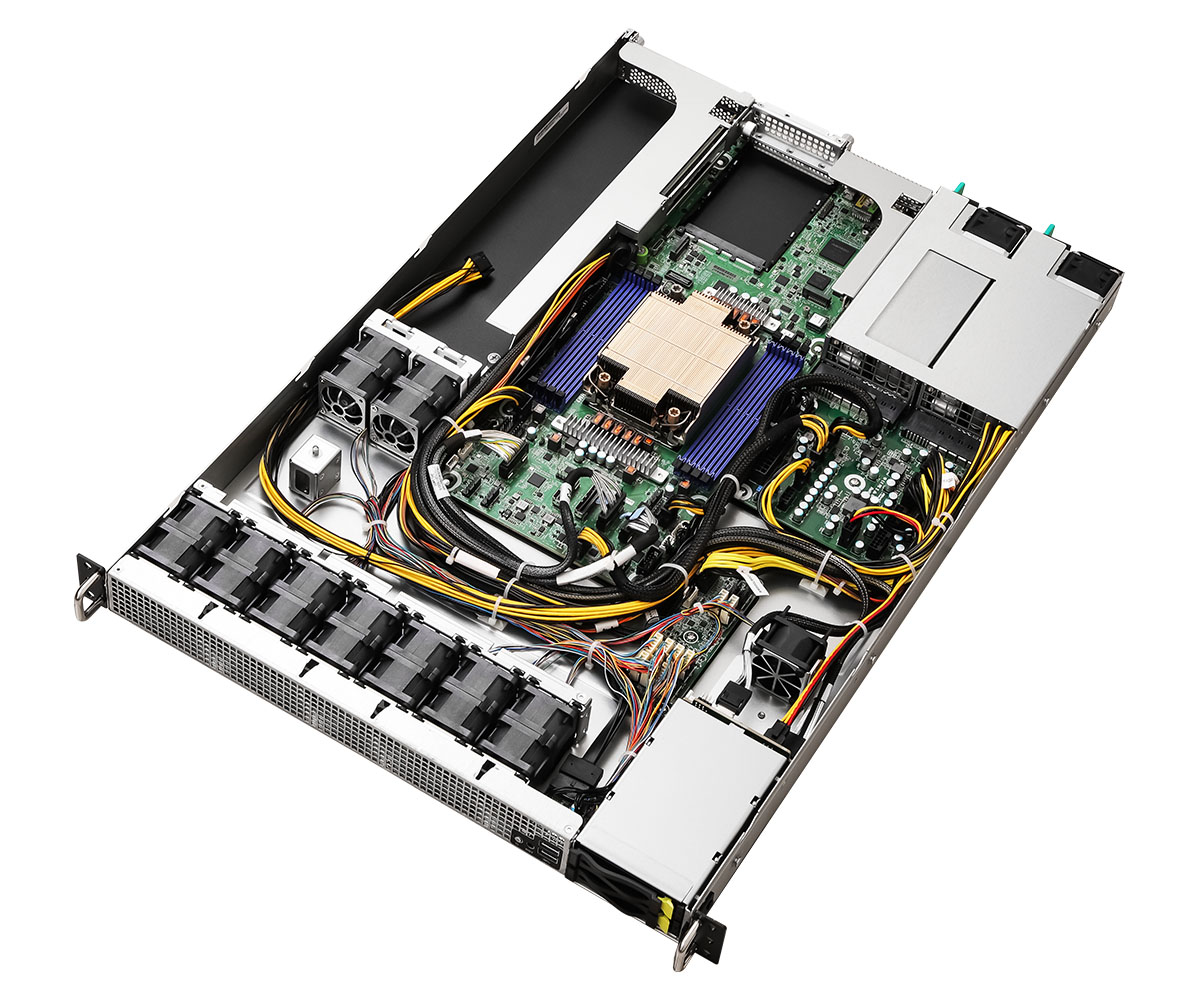
NEW
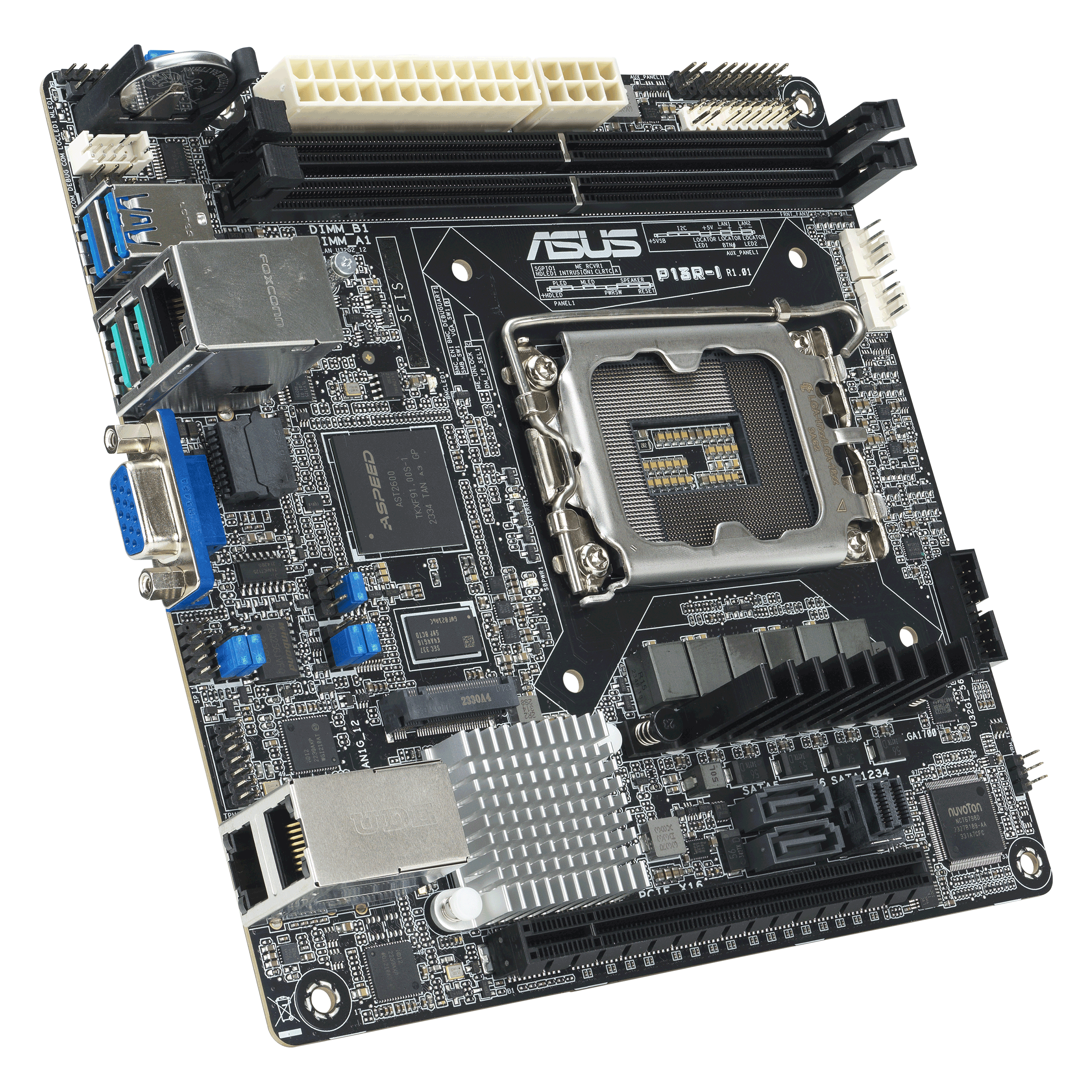
NEW

4U UP フロント/リアローディングストレージサーバー、36基のホットスワップ対応3.5インチSAS/SATAベイ(SAS HBA搭載)、PCIe 5.0対応
NEW

2U UP フロントローディング型ストレージサーバー12基の直接接続ホットスワップ対応3.5インチSAS/SATA/NVMeベイ(SAS HBA搭載)PCIe 5.0対応
NEW

2U UP フロントローディング型ストレージサーバー12基の直接接続ホットスワップ対応3.5インチSAS/SATA/NVMeベイ(SASハードウェアRAID)搭載PCIe 5.0対応
NEW

1Uショートディープ 14世代 Intel Core エッジサーバー(内蔵2.5インチドライブベイ×2、PCIe 5.0搭載)
NEW

1U 14世代インテル Core エッジサーバー(外部2.5インチドライブベイ×6基、PCIe 5.0搭載)
NEW

X14 2UコンパクトリアI/OエッジAIシステム(最大7基のPCIe 5.0スロット搭載、冗長AC/DC電源ユニット付き)
NEW

最大10基のPCIe 5.0 x16スロット+最大14基のE1.S NVMeドライブを搭載した3U Intel DP Edgeデータセンターシステム
NEW

インテル® Core™ Ultra プロセッサー搭載 Mini-ITX 組込みシステム
NEW

インテル Xeon D-1749NT プロセッサ搭載の組み込み型 Mini-ITX ボックス
NEW

インテル Xeon D-1736NT プロセッサ搭載の組み込み型 Mini-ITX ボックス
NEW

インテル Xeon D-1718T プロセッサ搭載の組み込み型 Mini-ITX ボックス
NEW
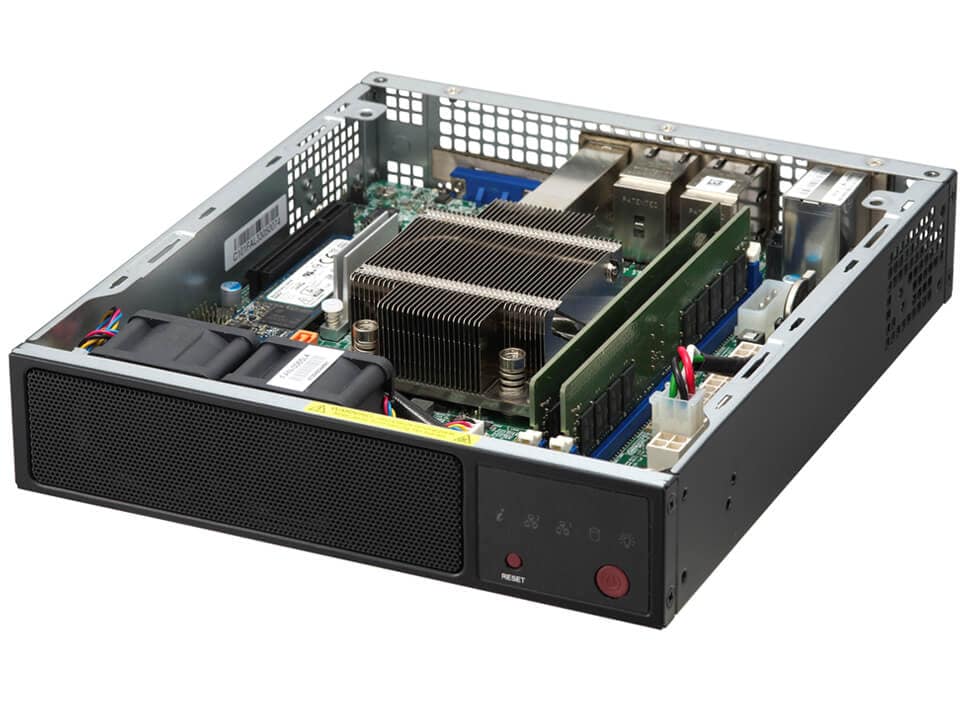
8コアIntel AtomプロセッサC5000 SoC搭載の組み込み型Mini-ITXボックス
NEW
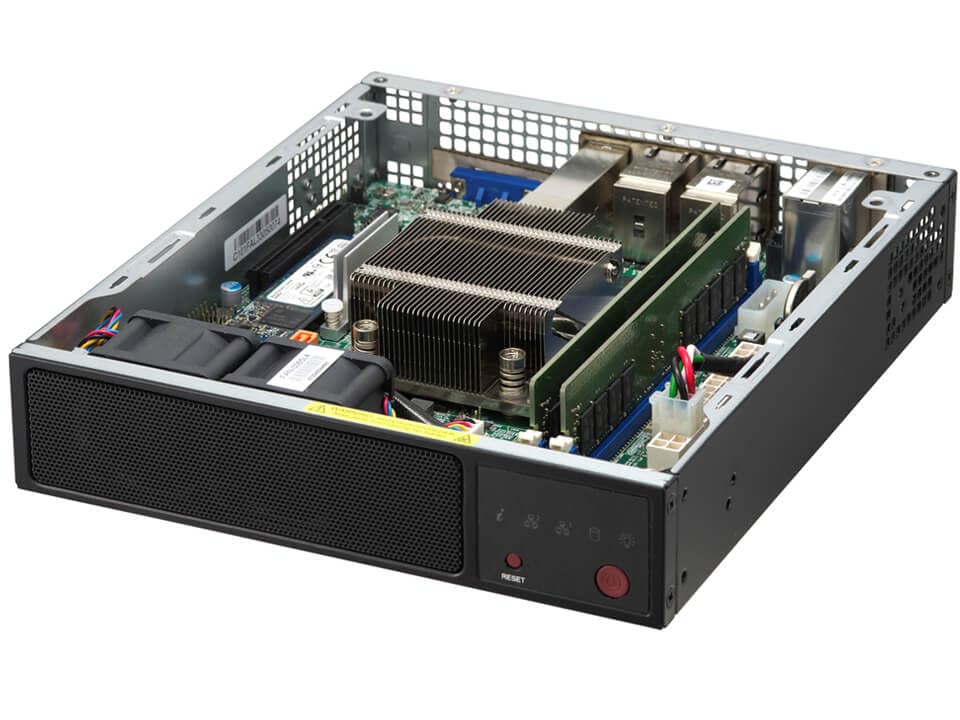
4コアIntel AtomプロセッサC5000 SoC搭載の組み込み型Mini-ITXボックス
NEW

3.5インチIntel Raptor Lakeマザーボード搭載のコンパクト組込み型ボックスPC
NEW

3.5インチIntel Raptor Lakeマザーボード搭載のコンパクト組込み型ボックスPC
NEW

コンパクトな組み込みボックスPC(4コアCPU搭載)
NEW

8コアCPU搭載のコンパクトな組み込みボックスPC
NEW

第12世代インテル® Core™ プロセッサー搭載 ファンレス ミニITX 組み込みボックス
NEW

8コアCPU搭載のコンパクトなファンレス組込みシステム
NEW

コンパクトなファンレス組み込みシステム(4コアCPU搭載)
